Mass Spectroscopy – Understanding M+, M+1 and M+2 Peaks
Mass Spectroscopy – Understanding M+, M+1 and M+2 Peaks
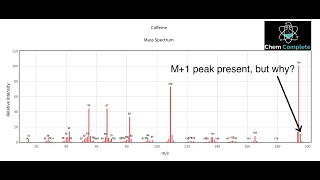
Parent Peak versus Base Peak in Mass Spec Lesson:
The Spectroscopy Solving Course: https://www.youtube.com/watch?v=bNkGMkzbBXI&list=PLpqnaa47Nj3DkYR3g8DcnSWjGMRi2enU-
Support the Channel!
Buy Unknown Spectroscopy Solving Guide Here, More Mass Spec Practice!: https://www.chemcomplete.com/product-page/solving-unknown-organic-structures-with-spectroscopy
Buy Other Guides Here:
https://www.chemcomplete.com/buy-guides
Customized tests with answer keys available as a service!
Need lab report help? Reach out to us!
Visit us Online: https://www.chemcomplete.com/
Other ways to make one time donations:
paypal.me/chemcomplete15

Not in practical aspect
can we get M+3 peak in mass spectra and why?
but this doesnt explain why im gay
Very helpful
Easy to understand 🙂
oh my god! I can’t believe I followed the whole 12 minutes and understood it better than the whole semester worth of lectures! my professor is pretty useless but thankfully there is youtube to save the day. Thank you so much, sir, you wouldn’t understand the pain I’ve been struggling with. It’s so hard to follow lectures and listen so intensely and understand nothing. But here I am feeling so much better about the material. Everything is so clear and easy to understand! Thank you thank you thank you!!
Nice👍